Abstract
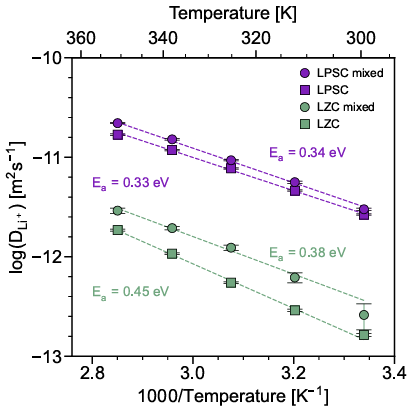
In the pursuit of long-lasting, high-energy density solid-state batteries, dual solid electrolyte designs offer a unique path toward stabilizing the electrode-electrolyte interfaces in the presence of a high-voltage positive electrode and a low-voltage negative electrode. However, electrolyte pairings that form a stable interface under battery operating conditions must be identified. In this study, we used the onset reaction temperature as a proxy to assess the chemical compatibility between a chloride (Li2ZrCl6 (LZC))catholyte (a high oxidative stability electrolyte) and two common thiophosphate (Li3PS4 (LPS) and Li6PS5Cl (LPSC)) anolytes (high reductive stability electrolytes). While LPS reacts with LZC starting at 90°C, the LZC–LPSC pairing appears stable up to 260°C. When heated to 300°C, the two pairings decompose to form LiCl, a layered LixZryP2S6 phase, ZrS2, ZrS3, S8, as well as γ−Li3PS4 for the LZC–LPSC pairing. Consistent with those findings, first-principles calculations show that neither pairing is thermodynamically stable. However, a higher decomposition energy is predicted for the LZC–LPSC combination, suggesting that it is kinetically stabilized up to 260°C. The LZC–LPSC interface is conductive to Li-ions, with an extremely low resistance (4.2 Ω cm−2). Furthermore, cells comprising an LZC–LPSC bilayer separating a LiNi0.8Mn0.1Co0.1O2 positive electrode and Li-In negative electrode exhibit exceptionally stable performance, with 90.0 % and 83.8 % of their initial capacity retained after 200 and 500 cycles, respectively. These findings allow us to propose several criteria for designing stable electrified interfaces for a wide range of electrochemical devices.