Abstract
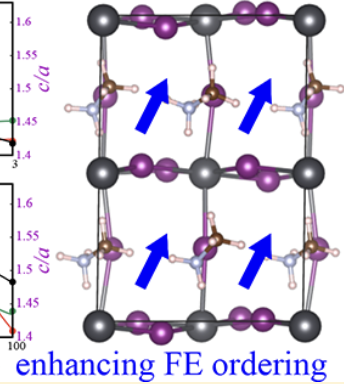
Organic–inorganic hybrid perovskites with a prototype formula MAPbI3 (MA = CH3NH3) have shown great promise in next-generation solar cells, yet a full understanding of their high power conversion efficiency relative to their inorganic counterparts has not been achieved. One of the most plausible arguments for their high efficiency is the ability of organic cations to form ferroelectric (FE) domains. By using first-principles calculations to examine the rotational behavior of MA cations in MAPbI3, here we show a relationship between the lattice structures and the FE dipole ordering of MA cations. It is found that the MA cations could form a spontaneous FE dipole ordering in tetragonal MAPbI3 at room temperature. The tendency of the FE formation is strongly related to the ratio of lattice parameters of MAPbI3. On the basis of the developed structure–ferroelectric-property relationship, we propose that a biaxial or uniaxial compressive strain and an anion doping with small halogen ions can further enhance the FE dipole ordering. These findings are in good agreement with the experimental discoveries that high-performance solar cells always incorporate mixed halide hybrid perovskites involving Br or Cl ions. This work may provide some guidelines for rational designs of highly efficient hybrid perovskite solar cells.