Abstract
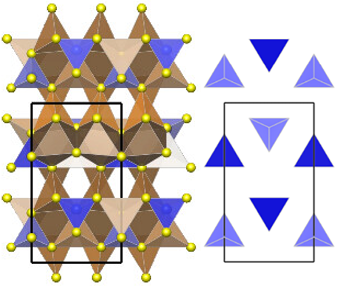
Thio-lithium superionic conductors (thio-LISICONs) are a family of promising solid electrolyte materials for potential applications in solid-state batteries. The orthorhombic polymorph of the thio-LISICON Li4SiS4 (o-Li4SiS4) has been known for decades, but its complete crystal structure has been reported only recently. Here, using single-crystal X-ray diffraction, we reevaluated the crystal structure of o-Li4SiS4 and showed that o-Li4SiS4 crystallizes in space group Pmn21 (no. 31, a = 7.7694(15) Å, b = 13.731(3) Å, and c = 6.1413(12) Å). The crystal structure of o-Li4SiS4 consists of isolated SiS4 tetrahedra arranged in a zigzag-type manner, whereas Li atoms are coordinated both tetrahedrally and octahedrally by sulfur atoms of the SiS4 groups. Structures identified by first-principles calculations support the lower symmetry solution presented here, with the Pmn21 polymorph being more stable at room temperature than a higher symmetry phase. By knowing the accurate crystal structure of o-Li4SiS4, we investigated the solid solution behavior with another group IV thio-LISICON, Li4SnS4. Rietveld refinements of powder X-ray diffraction data revealed the solid solution Li4Si1-xSnxS4 (0 ≤ x ≤ 1, Δx = 0.1), which shows a nearly ideal Vegard-type behavior for all silicon-containing samples. 29Si and 119Sn magic-angle-spinning solid-state NMR and Raman spectroscopy showed the presence of SiS4 and SnS4 tetrahedral moieties, with the spectra showing expected behavior consistent with the silicon–tin ratio in the materials. Electrochemical impedance spectroscopy revealed the highest ionic conductivity of 8.4 × 10-6 S cm-1 at 25 ℃ for Li4Si0.5Sn0.5S4, accompanied by the lowest migration barrier of ~0.37 eV.